- About ACNS
- Meetings
- Education
- Practice
- Research
- Advocacy
- Membership
Contributed by:
Tatsuya Oishi, MD and Ruple S. Laughlin, MD
Mayo Clinic Rochester
A healthy 21-year-old male patient with no relevant past medical history presented to our clinic for evaluation of a slowly progressive, painless right leg weakness. He first noted right calf atrophy and occasional fasciculations in the right foot sometime in his early teenage years, which progressed to a right foot drop. When he underwent a local evaluation about 4 years ago, his weakness was attributed to chronic inflammatory polyradiculoneuropathy; however subsequent treatment with steroids did not yield any benefit. Review of systems, medications, family history, and social history were unrevealing. On examination, the right anterior tibialis muscle was visibly atrophied, and he had moderate-to-severe weakness of right foot dorsiflexion, inversion, eversion, as well as toe flexion and extension. He had normal strength in his proximal right lower limb and the remainder of the body. The right Achilles reflex was absent, while the right patellar reflex and muscle stretch reflexes elsewhere were preserved. Sensory exam was unrevealing in the right leg as well as elsewhere throughout the body.
Given this focal weakness, an electrodiagnostic study was requested. Routine lower limb nerve conduction studies (NCS) demonstrated reduced right fibular and tibial compound muscle action potential (CMAP) amplitudes, with normal F-wave latencies and without conduction block or temporal dispersion (Figure 1; Table 1). Right sural sensory nerve action potential was also reduced, while the right medial plantar sensory response was absent; superficial fibular sensory nerve action potential (SNAP) was not performed. For comparison, all left lower limb NCS were within normal limits. Right lower limb needle electromyography demonstrated long duration, high amplitude motor unit potentials in distal sciatic nerve innervated muscles (anterior tibialis, gastrocnemius, and peroneus longus; Table 2). Of these, fibrillation potentials were seen in the gastrocnemius and anterior tibialis. The long head of biceps femoris had mildly high amplitude motor units, but otherwise appeared normal. Electromyography of the short head of biceps femoris as well as proximal L5 and S1-root innervated muscles (tensor fascia lata, L5 paraspinal muscle, and gluteus maximus) were unremarkable.
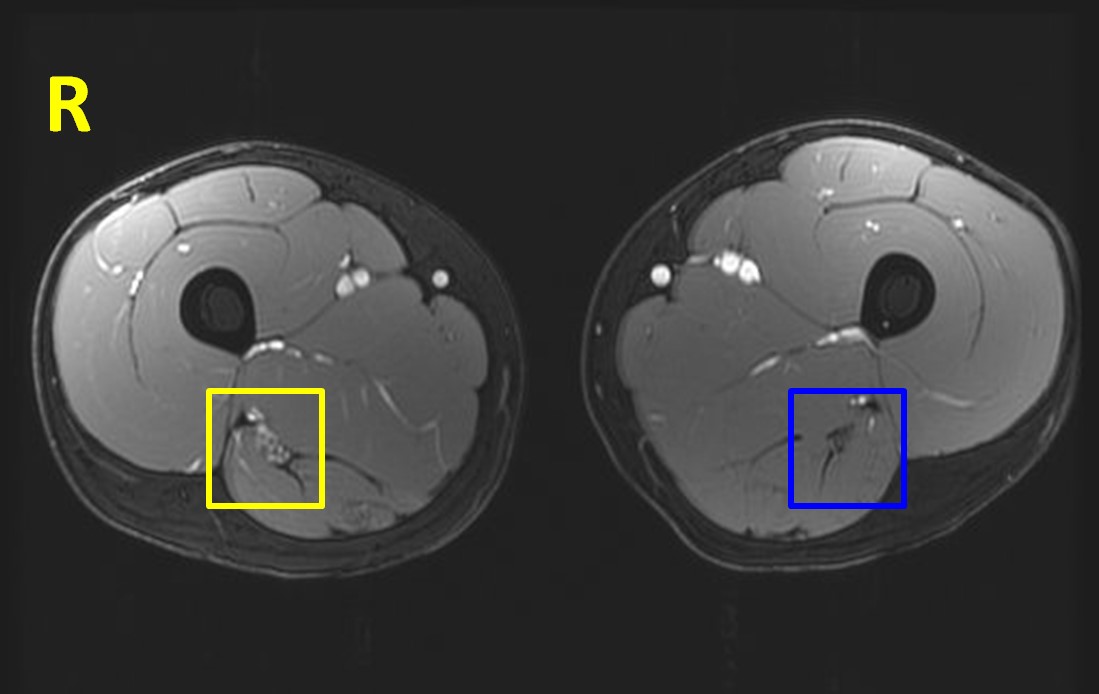
MRI of the lumbar plexus and lower limbs demonstrated a longitudinally extensive, smooth concentric enlargement of the right sciatic nerve, extending from proximal thigh to the popliteal fossa (Figure 2). The enlargement had features of T2-signal hyperintensity, contrast enhancement, and apparent individual internal fascicles.
Figure 1.
Right (yellow) and left (blue) fibular motor responses. CMAP amplitude from stimulation at ankle was 0.73 mV; there is <10% difference between proximal and distal amplitudes.
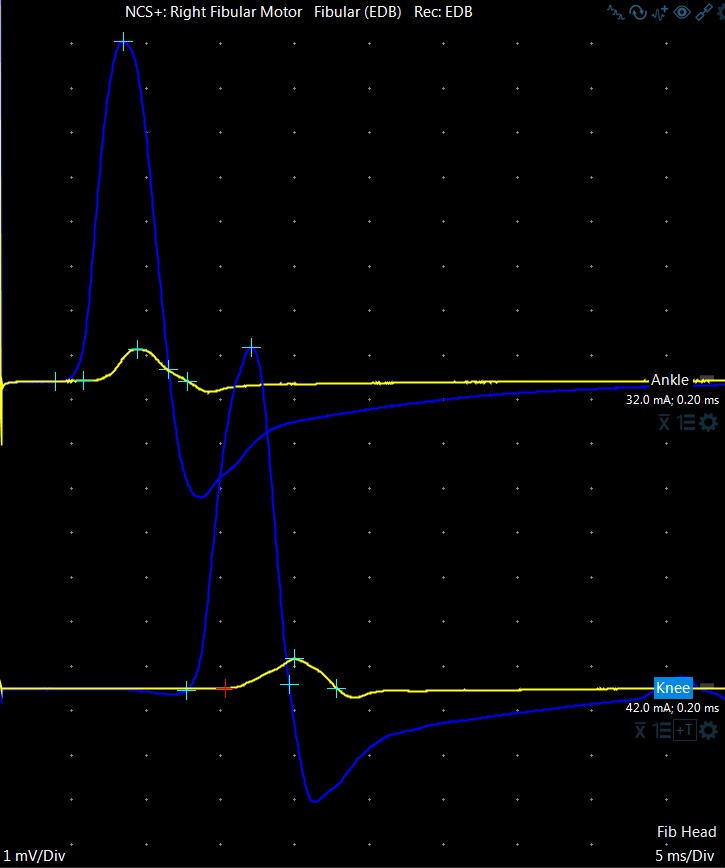
Table 1:
Right and left lower limb routine NCS. Abnormal values are displayed in bold. Normal lab values are displayed inside parentheses.
| Amplitude (mV) | Conduction velocity (m/s) | Distal latency (ms) | |
| Right tibial motor | 1.5 (> 4.0) | 45 (> 40) | 5.3 (< 6.1) |
| Right fibular motor * (fig 1, yellow) | 0.7 (> 2.0) | 41 (> 41) | 5.8 (< 6.6) |
| Left fibular motor* (fig 1, blue) | 7.7 (> 2.0) | 47 (> 41) | 3.9 (< 6.6) |
| Right medial plantar sensory | No response | ||
| Left medial plantar sensory | 31 (> 7.0) | - | 3.1 (< 4.0) |
| Right sural sensory | 6 (> 6.0) | 40 (> 40) | 4.7 (< 4.5) |
| Left sural sensory | 21 (> 6.0) | not measured | 3.8 (< 4.5) |
| * Fibular CMAPs were recorded from extensor digitorum brevis muscle, with distal stimulation site at the ankle and proximal stimulation site at the knee. Superficial fibular SNAP, and fibular CMAP with recording from anterior tibialis muscle were not performed. | |||
Table 2:
Result of standard concentric needle electromyography, completed in the right lower limb. Severity of each finding is rated from mild (+) to severe (++++); normal findings are left blank. Estimated occurrence of polyphasic units are ranked from <15% (left blank) to 100%. No fasciculation potentials were witnessed.
| Fibrillations | Units | Recruitment | Duration (long) | Amplitude (high) | Phases | Turns | |
| Tibialis anterior | + | abnormal | + | ++ | ++ | 15% | + |
| Gastrocnemius (medial head) | ++ | abnormal | ++ | ++ | ++ | 25% | ++ |
| Peroneus longus | none | abnormal | + | ++ | ++ | normal | normal |
| Biceps femoris (long head) | none | mildly abnormal | normal | normal | + | normal | normal |
| Biceps femoris (short head), vastus medialis, tensor fasciae latae, gluteus maximus, and L5 paraspinal | none | normal | |||||
Figure 2.
Patient’s axial MRI of the bilateral thighs (SPGR sequence). Note the apparent enlargement and hyperintensity of the right sciatic nerve (outlined in yellow) compared to the left (in blue).
Question 1: Pathology will most likely show:
Question 2: Given the clinical history and findings, a biopsy would be: